Unveiling the Molecular Mechanism of Mitochondrial tRNA m3C Methylation by METTL8
Key Highlights :
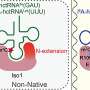
A study published in the journal Science Bulletin and led by Profs. Xiao-Long Zhou and En-Duo Wang (CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences) has shed light on the molecular mechanism of mitochondrial tRNA m3C methylation by the enzyme METTL8.
Transfer RNA (tRNA) is a key adaptor molecule in mRNA translation, and contains a large number of post-transcriptional modifications, such as 3-methylcytosine (m3C) modification, which regulates the speed and fidelity of protein synthesis. Previous studies have found that the m3C modification of human cytoplasmic tRNAs was mediated by METTL2A/2B and METTL6, while that of human mitochondrial tRNA Thr (hmtRNA Thr) and tRNA Ser (UCN) (hmtRNA Ser (UCN)) is catalyzed by METTL8. Human METTL8 generates two protein isoforms of different lengths by alternative splicing of mRNA, namely, METTL8-Iso1 and METTL8-Iso4.
To understand the molecular mechanism of mitochondrial tRNA m3C methylation, the researchers confirmed the conservation of the N-terminal extension (N-extension) of METTL8-Iso1 through multiple sequence alignment. In vitro enzyme activity determination revealed that METTL8-Iso4 had no m3C modification activity. They further proved that the N-extension of METTL8-Iso1 acted as a key tRNA-binding element in the catalytic process. Two completely conserved amino acid residues in all METTL2A/2B/8 proteins were identified. METTL8-Iso1 was able to mediate m3C32 modification for both cytoplasmic and E. coli tRNAs, which was not reliant on t6A37. However, cytoplasmic m3C32 modification enzymes METTL2A and METTL6 were unable to catalyze m3C32 modification of mitochondrial tRNA, indicating that METTL8-Iso1 has a more relaxed substrate specificity. The m3C32 modification did not affect the t6A37 modification and aminoacylation levels of hmtRNA Thr. Finally, they also revealed that METTL8-Iso1 interacted with mitochondrial seryl-tRNA synthetase (SARS2) and mitochondrial threonyl-tRNA synthetase (TARS2), respectively, and significantly promoted aminoacylation activity of SARS2 and TARS2.
In summary, this study revealed the molecular mechanism of mitochondrial tRNA m3C biogenesis mediated by METTL8, which relies on a specific N-extension as a key RNA-binding element. METTL8 had a broad spectrum of heterogenous tRNA substrates, which provided a basis for preparation of tRNAs containing only a m3C moiety. This work provides a comprehensive understanding of the conservation and difference between cytoplasmic and mitochondrial tRNA m3C modification.
The findings of this study are of great importance for understanding the molecular basis of protein synthesis. It provides insight into the regulation of mitochondrial tRNA m3C methylation and can be used to design effective strategies for tRNA modification and protein synthesis regulation.
